Diving Deep into High-Level Disinfection vs. Sterilization
Incision · · 4 min readWelcome back to our series on the inner workings of the Sterile Processing Department (SPD). In our previous article, "Sterile Processing and the Operating Room: Why Patient Safety Can't Be Rushed", we emphasized the importance of embracing the 4.5-hour processing time that is sometimes needed to ensure patient safety and care. Today, let's up the ante and delve into a process that often provokes debate within SPD circles: High-Level Disinfection (HLD).
The Deep Dive: A HLD Overview
For the uninitiated, HLD is a procedure that kills all microorganisms and inactivates some, but not all, bacterial spores. In layman's terms? It's akin to an extreme spring cleaning, but not quite a home makeover. HLD is generally reserved for semi-critical medical devices—those that come into contact with mucous membranes but don't penetrate sterile tissues.

Pre-Cleaning: Skimming the Surface
In SPD, the journey into effective High-Level Disinfection begins with decontamination. This step involves the physical removal of soil from medical devices like flexible endoscopes, ensuring that subsequent disinfection stages are effective. Miss a spot here, and the whole process can be compromised.

Disinfectant Immersion: Into the Depths
This involves submerging the item in a high-level disinfectant like glutaraldehyde or ortho-phthalaldehyde for a specific time frame as determined by the manufacturer. This process should be performed by automated machines but may be performed manually when automated options are not available. During this phase, it’s essential to ensure that the disinfectant makes contact with all surfaces for the full duration recommended.


Rinsing and Drying: Resurfacing
Rinsing and drying are not mere afterthoughts but essential final steps to remove any residual disinfectant and mitigate bacterial recontamination.
The Great Debate: HLD vs. Sterilization
The Case for HLD
Advocates for HLD emphasize its efficiency and practicality, particularly in high-throughput healthcare environments where sterilization is not required For instance, the time required for High-Level Disinfection is typically less than that for sterilization processes. This can be crucial in situations where quick turnaround times are essential for instrument reprocessing. Furthermore, certain materials that are sensitive to the high temperatures and pressures of common sterilization methods can be safely reprocessed using HLD, thus extending the lifespan of some medical devices.
Efficiency gains are not merely operational; they can lead to substantial cost savings by reducing the need for single-use instruments or additional instrument inventory to accommodate longer sterilization cycles.
The Case Against HLD
However, the arguments against using High-Level Disinfection as a substitute for sterilization are backed by heavyweights in the field, including the CDC and AAMI. Sterilization methods, such as steam, ethylene oxide (EtO) gas, and forms of vaporized hydrogen peroxide, have the capacity to eliminate all forms of microbial life, including hardy bacterial spores. HLD, while effective against a broad spectrum of microorganisms, may not reliably eliminate bacterial spores. This makes sterilization the only method for reprocessing critical surgical instruments that come into contact with sterile body tissues. Not sure if mucous membranes will be intact or not? Sterilization is the standard to ensure a patient’s safety.
Another compelling argument against HLD is the potential for human error. The effectiveness of HLD is highly dependent on strict adherence to protocols, including contact time and concentration, leaving room for variability and risk.
Key Takeaways
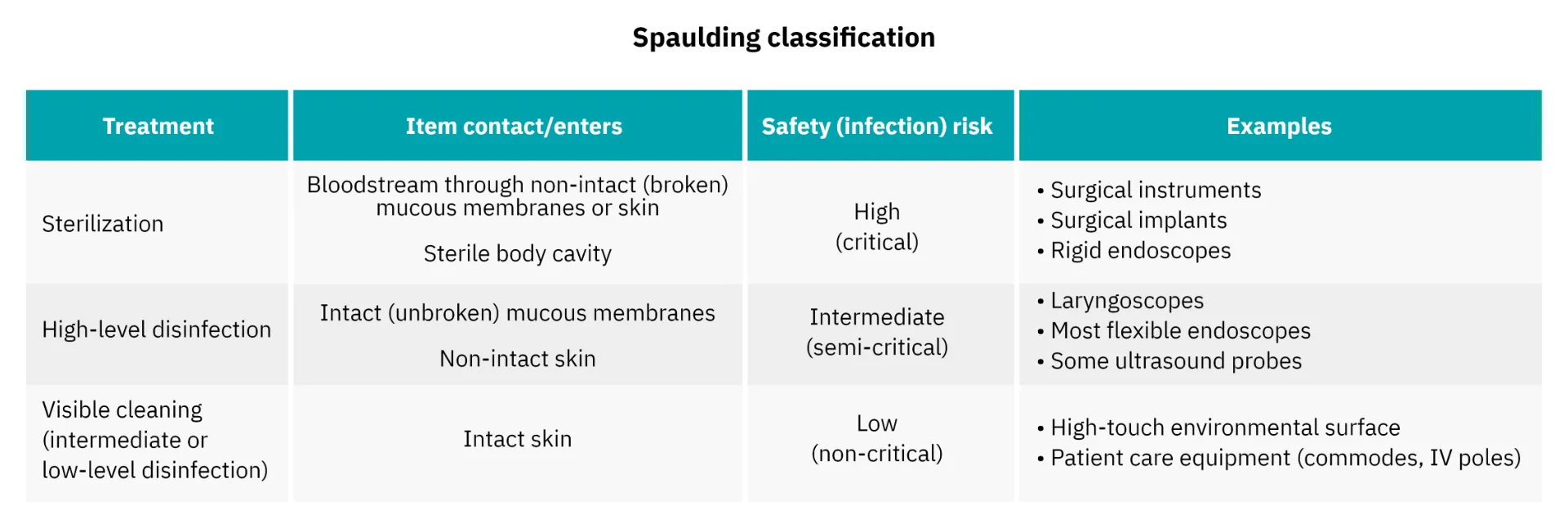
- Differentiating HLD and Sterilization: Understand the scope and limitations of each.
- Adhere to Best Practices: Regulatory bodies like the CDC and guidelines like AAMI provide crucial frameworks.
- Efficacy and Risk: Weigh the pros and cons of reprocessing with HLD or sterilization, follow the IFU and err on the side of patient safety.
Coming Next: Bioburden and Biofilm
Dive deeper with us into the world of bioburden and biofilm in our upcoming article. You won't want to miss this critical discussion.
Sterile Processing is a field that never remains static, and neither should our understanding of it. Continue to dive deep with us into these critical topics that form the backbone of patient safety in surgical settings. If you haven't had the chance to read our previous article, "Sterile Processing and the Operating Room: Why Patient Safety Can't Be Rushed", it's well worth your time for a comprehensive understanding of sterilization processes.
Here at Incision, we’re dedicated to writing blogs that inspire conversation, bring teams closer together and educate in a dynamic way. When we learn together we grow together, so join the conversation!